- Home
- Blogs
COVID-19 – the distinction between vaccines and vaccination
Browse this blog post
Related news and insights
News: 09 February 2024
Allen & Overy advises Sartorius group on EUR1.4bn equity raising
Publications: 13 December 2023
Blog Post: 21 November 2023
Blog Post: 16 May 2023
Webinar: Regulatory interplay between medical devices and medicines - EU and U.S. hotspots
Vaccines
With thanks to a huge global scientific effort, we now have several COVID-19 vaccines that have met the standards of safety and efficacy set by regulators.
In the UK, Regulation 174 of the Human Medicines Regulations 2012 (HMRs) allows for the temporary authorisation of the supply of an unlicensed medicine in the event of a public health emergency. The three vaccines that were initially temporarily licensed under Regulation 174 have now all been granted a conditional marketing authorisation by the UK’s Medicines and Healthcare products Regulatory Agency (MHRA), together with a fourth vaccine. The same four vaccines have been granted a conditional marketing authorisation by the European Medicines Agency (EMA). The US Food and Drug Administration (FDA) and Japan’s Pharmaceuticals and Medical Devices Agency (PMDA) have each approved three of the four vaccines under their equivalent emergency use procedures.
The World Health Organization (WHO) emergency use listing (EUL) also has a procedure for assessing and listing unlicensed vaccines and therapeutics aiming to speed up their availability in public health emergencies. There are now ten vaccines approved by the WHO for emergency use:
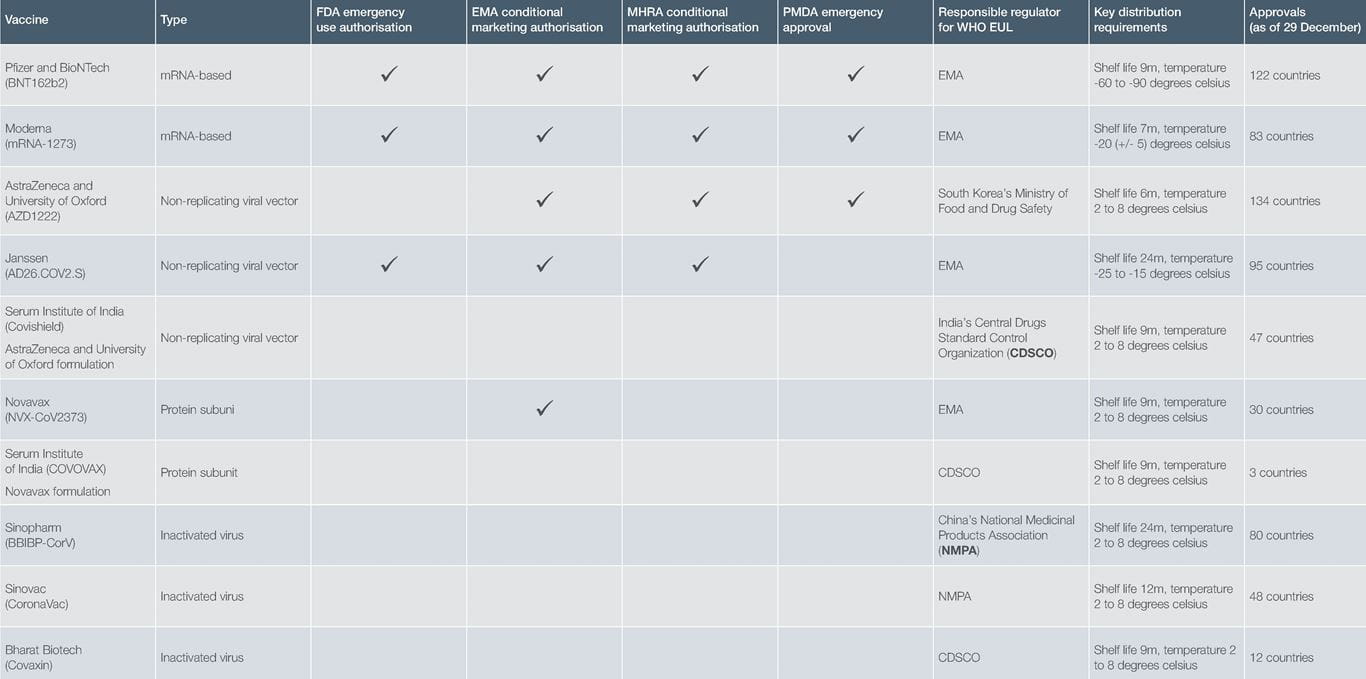
As well as these vaccines, there are many more on the way – as of 28 December 2021, there were 137 vaccines in clinical development and 194 vaccines in pre-clinical development. Although the majority are injectable (84%), two dose (61%) vaccines, they are based on a wide range of different platforms – protein subunit (35%), RNA (17%), non-replicating viral vector (15%), inactivated virus (13%) and DNA (11%).
Vaccination
In the UK, as of 28 December 2021, over 51 million and 47 million people have received a first and second dose respectively. In addition, over 33 million people have received a booster dose. This equates to about 82.3% of the UK population (over aged 12) being double jabbed, with 57.5% also having had a booster. Many countries have also carried out successful vaccination programmes and fully vaccinated over 70% of their population, including Germany, France, Italy, Spain, Portugal, Finland, Sweden, Norway, Denmark, Singapore, China, Japan, the UAE, Australia and New Zealand. Unfortunately, the success of the vaccination efforts in these countries has not been capable of replication worldwide.
As I mentioned previously, new Regulation 247A of the HMRs allowed a wider range of personnel to administer vaccines and, due to this change, I have the privilege of being involved in the NHS Covid-19 vaccination programme as a St John Ambulance volunteer vaccinator. One thing that has particularly struck me is the amount of planning, and additional people and resources, needed to deliver a safe and efficient mass vaccination programme.
As of 13 December 2021, only 20 African countries had fully vaccinated at least 10% of their population. Only six of those countries have hit the year-end target of fully vaccinating 40% of their population. Only two countries (Mauritius and the Seychelles) have reached the 70% threshold that is considered necessary. Although many African countries are successfully building on their previous experiences of vaccination campaigns (for example, measles, polio and ebola), many do not have the funds needed to cover operational costs and many face shortages of staff and necessary commodities.
Globally, there has now been over 280 million confirmed cases of COVID-19 and nearly five and a half million reported deaths. Higher-income countries and global organisations must step up and ensure lower-income countries can address the specific challenges they face to deliver successful vaccination programmes. Not just for morality, but also to prevent the virus being given space to mutate – possibly into more dangerous variants. As the WHO has said consistently - ‘it’s not vaccines that will stop the pandemic, it’s vaccination.’
Sources:
World Health Organisation
GOV.UK (Coronavirus)
World Health Organization - Africa
WHO Coronavirus (COVID-19) Dashboard
COVID-19 Vaccine Tracker
World Health Organization - Regulation and Prequalification
European Medicines Agency
U.S. FDA
Allen & Overy - Covid-19 coronavirus – the UK’s preparation for a vaccine
UK Health Security Agency
BBC News
World Health Organization - Africa
